Molecular Aggregates in Stable Aqueous Three-Phase Surfactant Systems and Their use in Producing CdS Nanowires
Abstract
Aqueous three-phase surfactant systems (A3PS) are important, multicomponent, stable three-phase equilibria with coexisting forms in a common colloid solution, but have been largely ignored regarding further characterization and application. Mixing simple, commercially available, single-tailed anionic/nonionic or anionic/cationic surfactants in water can spontaneously produce stable A3PS with coexisting multiscale self-assembled structures including discs, lamellas, micelles and vesicles. As with conventional aqueous two-phasesystems (A2PS), A3PS can be applied in partition and extraction processes. Here, the A3PS was also used as a mild media for one-step synthesis of multiscale CdS nanowires. Particularly, the A3PS does not change and simultaneously separates the CdS nanowires with the comparable size in one phase, which provides a facile strategy for collection of monodisperse nanomaterials. We expect that this present work can expand recognition of A3PS for use in theoretical and applied studies.
Introduction
Molecular self-assembly, via non-covalent interactions, has been recognized as a fascinating, practical, bottom-up approach to achieve stable, structurally well-defined, and functional objects at the nano- and microscale in biological systems and materials synthesis. While a large number of self-assembled forms such as micelles, discs, vesicles, tubes, and fibers have been reported in aqueous solutions of natural or synthetic amphiphiles, the future development of self-assembly should see a move towards the construction of variably-scaled multicomponent complex systems with diverse function.
Aqueous multiphase separation is an interesting assembly process in colloid science that results in a higher degree of complexity in the system but can be readily achieved by a variety of easily accessible conditions and components. In particular, aqueous two-phase systems (A2PS), introduced by the pioneering work of Albertsson, have been acknowledged to develop biotechnical processes for the recovery and purification of many biological materials including proteins, nucleic acids, and cells. A conventional A2PS consists of two incompatible hydrophilic polymers, or a hydrophilic polymer and a salt, with water as continuous medium. Each of the phases obtained is enriched in one of the components. In addition, liquid-liquid phase separation upon heating above component cloud point is a common phenomenon in dilute nonionic surfactant solutions, where one phase becomes rich in surfactant and the other poor. In recent years, many new A2PSs, including anionic/nonionic and anionic/cationic (catanionic) surfactants, have been designed for applications in separations and purification processes.
In contrast, there has been no special exploration of aqueous three-phase surfactant systems (A3PS) for separations or other applications. In fact, the previous aqueous three-phase systems were also formed by mixing nonionic surfactants and two types of polymers in water. At present, the A3PSs have been frequently reported in phase diagrams of pure surfactant solutions. Hoffmann et al.determined some three-phase regions such as micelle/micelle/lamella (L1/L1/Lα) and lamella/sponge/micelle (Lα/L3/L1) in aqueous solution of tetradecyldimethylamine oxide and medium alcohol. Other surfactant works also reported the lamella/sponge/micelle (Lα/L3/L1) three-phase in sodium bis(2-ethyl hexyl)sulfosuccinate/NaCl/H2O systemand reversed micelle/lamella/hexagonal structure (L2/Lα/H1) three-phase in tetraethylene glycol monododecyl ether/sucrose ester/decane/H2O system. However, the aggregate details of these three-phases have not been confirmed by direct observation until now. For example, it is attractive to confirm the differences between the two separated micellar phases in the above L1/L1/Lα solution. Therefore, the confirmation of complex self-assembled forms, the establishment of simple three-phase models, and the characterization of important physicochemical properties for A3PS are significant, timely issues. Furthermore, as a coexisting multiphasic equilibrium system with rich complexity, A3PS can exhibit higher partition efficiency for biomaterials or other species. As might be expected, the present lack of a general method for the production of A3PS has hindered its development and application.
To help generalize the characterization and modification of A3PS properties, we report here aqueous mixtures of anionic/nonionic and anionic/cationic surfactants that produce aqueous three-phase systems: (i) sodium dodecylethoxysulfonate (CH3(CH2)11(CH2CH2O)2.5SO3Na, Texapon N70)/tetradecyldimethylamine oxide (C14DMAO)/formic acid (HCOOH), (ii) lauric acid (CH3(CH2)10COOH, LA)/tetraethylene glycol monododecyl ether [CH3(CH2)11(CH2CH2O)4OH, C12EO4], and (iii) sodium laurate (SL)/tetradecyltrimethylammonium bromide (TTABr). Using system (i) as a model system, we also present the application of A3PS for the solubilization and partition of dyes, and one-step synthesis and separation of multiscale CdS nanowires in one common three-phase solution.
Results
After the three surfactant systems were kept at constant temperature for one month, two clearly visible liquid-liquid phase boundaries were observed separating each system into three aqueous phases. Sample photographs, taken after 12 months, revealed that the apparent phase behavior remained without obvious change, implying that these A3PSs are stable. A common feature of the three systems is that one of their phases shows significant birefringence under crossed polarizers while the other two phases are transparent and lack birefringence (Fig. S1). Each phase of the three samples takes up a different phase volume fraction. These preliminary observations led us to question how the surfactant mixtures are distributed in the three aqueous phases, and what complex self-assembled forms exist in these three phase systems.
A3PS in anionic/nonionic system of Texapon N70/C14DMAO/HCOOH/H2O
Cryogenic transmission electron microscopy (cryo-TEM), dynamic light scattering (DLS), elemental analysis, density and volume fraction measurements, and pH measurements were employed to determine the inherent properties of the A3PS. As expected, the system (i) exhibits typical behavior of an anionic/cationic system. Because the weakly basic, nonionic C14DMAO can be easily protonated by HCOOH, ion pairs form through the electrostatic interaction between C14DMAOH+(C14DMAO + HCOOH → C14DMAOH+ + HCOO−) and the sulfate group of Texapon N70mM C14DMAO as a function of the concentration of Texapon N70with equimolar concentrations of HCOOH at 25°C is shown in Fig. S2. Previously, it was observed that increasing Texapon N70 and formic acid concentrations resulted in a series of phase transitions. In this system, the three-phase region exists in a very narrow concentration region of 26 to 26.8 mM Texapon N70.
Fig. 1a shows the optical photographs of three-phase samples with and without crossed polarizers. In the top phase of a sample of 26.5 mM Texapon N70/100 mM C14DMAO/26.5 mM HCOOH, at Texapon N70/C14DMAO molar ratio of 0.25 determined by elemental analysis (Table 1), one observes monodisperse nanodiscs with a mean diameter of 12 ± 3 nm coexisting with a few flexible bilayer fragments of over 100 nm in length (Fig. 1b). Coexistence of different microstructures of differing size can be easily identified by DLS, as shown in Fig. 2, for the top, middle, and bottom phases of this system at scattering angle of 90°. The hydrodynamic radius (Rh) obtained by CONTIN analysis confirmed the presence of two different aggregates in the top phase with mean sizes of 10 nm and 150 nm that may be assigned to the nanodiscs and bilayer fragments, respectively. The isolated nanodiscs lie with an edge-on orientation and appear as dark lines with tapered ends in the cryo-TEM images. The calculated pH value of a 26.5 mM formic acid aqueous solution is about 2.68, however, the pH was measured to be 5.75 ± 0.05 in the three-phase solution, indicating that most of formic acid reacted with the weakly basic C14DMAO to form C14DMAOH+. The presence of a small amount of H3O+ in solution indicates an incomplete protonation of C14DMAO; therefore, the nanodiscs possess a small excess of basic surfactant, giving the nanodisc edges anionic characte.
Figure 1: Molecular aggregates of A3PS in Texapon N70/C14DMAO/HCOOH/H2O at 25°C.
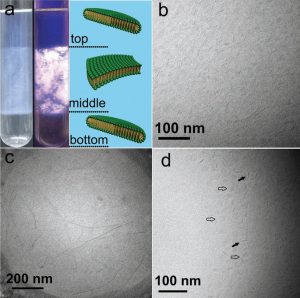
(a) Optical photographs of three-phase samples with and without crossed polarizers. Cryo-TEM images of nanodiscs, distorted bilayers, and nanodiscs in the top (b), middle (c), and bottom (d) phases, respectively. Open and filled arrows indicate discs in face-on and edge-on orientations, respectively (b).
Figure 2: DLS CONTIN plots of Texapon N70/C14DMAO/HCOOH/H2O system performed at scattering angle of 90°.
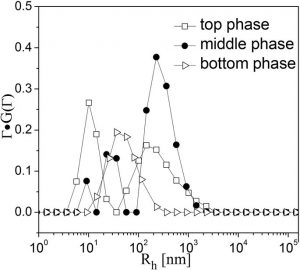
In the slightly turbid birefringent mid-phase at Texapon N70/C14DMAO ratios of 0.31, the cryo-TEM image shows very long bilayer fragments of several hundred nm (Fig. 1c). These lamellas are irregular, even distorted, and appear flexible. DLS results (Fig. 2) also indicate the polydispersion of these bilyer fragments that the average sizes are concentrated in about 10 nm, 30 nm and 300 nm, respectively. In the transparent bottom phase at Texapon N70/C14DMAO ratio of 0.32, a rearrangement of the surfactant results in a phase transition from large lamellas back to monodisperse, negatively charged nanodiscs with a mean diameter of 24 ± 4 nm (Fig. 1d). DLS also confirms their formation with a resulting meanRh of 35 nm (Fig. 2). In this phase some nanodiscs lie in an edge-on orientation and appear as dark bars (filled arrows in Fig. 1d), while discs in a face-on orientation appear as circles or as variably elongated ellipses (open arrows). Some of the observed differences in the morphologies of the discs results from the thickness of the vitrified aqueous film as prepared for cryo-TEM analysis.
From the top phase to the middle phase of the sample, the structure transforms from nanodiscs (L1‘) to large lamellas (Lα), accompanied with an increase in the Texapon N70/C14DMAO ratio, solution density, and phase volume fraction (Table 1). On the other hand, the bottom phase, also a nanodisc region (L1‘), possessed the highest Texapon N70/C14DMAO ratio and solution density, but the smallest volume. These macroscopic and microscopic changes reveal the structures and properties of the aqueous three-phase (L1‘/Lα/L1‘) that is related to the coexistence of complex aggregates and different surfactant compositions, as well as the resulting differences in solution density among the three phases. However, it is not known whether the aqueous three-phase formation is unique phenomenon in specific surfactant systems, or a more general phenomenon. In this system, excess salt is present with the two single-tailed anionic/nonionic surfactants. For comparison, the LA/C12EO4/H2O anionic/nonionic system was investigated to identify whether excess salt is a necessary for the formation of A3PS.
A3PS in salt-free anionic/nonionic system of LA/C12EO4/H2O
LA has a very low solubility in water, but dissolves in C12EO4 aqueous solution with a release of H+. Thus, the LA/C12EO4 mixture behaves as anionic/cationic mixture system because the oxygen atoms in C12EO4are partially protonated, resulting in a partial positive charge on the nonionic surfactant. The competition among the extra electrostatic interactions, the hydrophobic interaction and membrane undulation induces a rather rich phase behavior in the mixture. The phase diagram of the LA/C12EO4/H2O system at 25°C is shown in Fig. S3. The three-phase region is present in the range of WT = 10% to 30% and RLA = 0.225 to 0.275.
In a three-phase sample of LA/C12EO4/H2O system with WT = 23% andRLA = 0.25 (Fig. 3a), the clear upper phase has an obvious birefringence indicating the presence of anisotropic aggregate structures. This is confirmed by cryo-TEM images that show small unilamellar vesicles (Fig. 3b) with diameters ranging from 50 to 120 nm. The self-fusion of aggregates, where smaller vesicles were swallowed by larger vesicles or two vesicles merging together, was observed. In the three-phase systems, the pH was measured to be 3.30 ± 0.05. The excess H3O+ in solution indicates net negative charges on the membrane surface due to unpaired anionic surfactants. In the middle phase, the three-dimensional network structure of spherical and ellipsoidal objects (Fig. 3c), as the typical characteristic of the sponge L3 phase, was observed by the freeze-fracture TEM (FF-TEM). The sponge structure comprises highly interconnected bicontinuous bilayers. However, unlike the lamellar phase, the L3 phase presents no long-range order without static birefringence. Finally, compared with the slightly bluish top and middle phases, the bottom phase is clear and transparent, consisting of isolated nanodiscs ranging from 15 to 35 nm (Fig. 3d).
Figure 3: Molecular aggregates of A3PS observed in LA/C12EO4/H2O at 25°C.
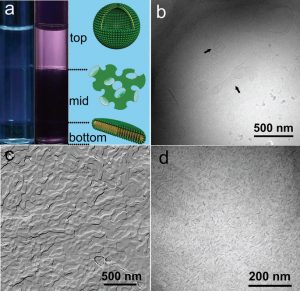
(a) Optical photographs of three-phase samples with and without crossed polarizers. (b) Unilamellar vesicles (50 to 120 nm in diameter) in the top phase. The arrows in image (b) show the fusion of small vesicles. (c and d) Sponge and nanodiscs (15 to 35 nm) in the middle and bottom phases, respectively.




